How Many Covalent Bonds Can Phosphorus Form - Web as such, the \(\ce{s}\) atom must have 12 valence shell electrons to form 6 covalent bonds.
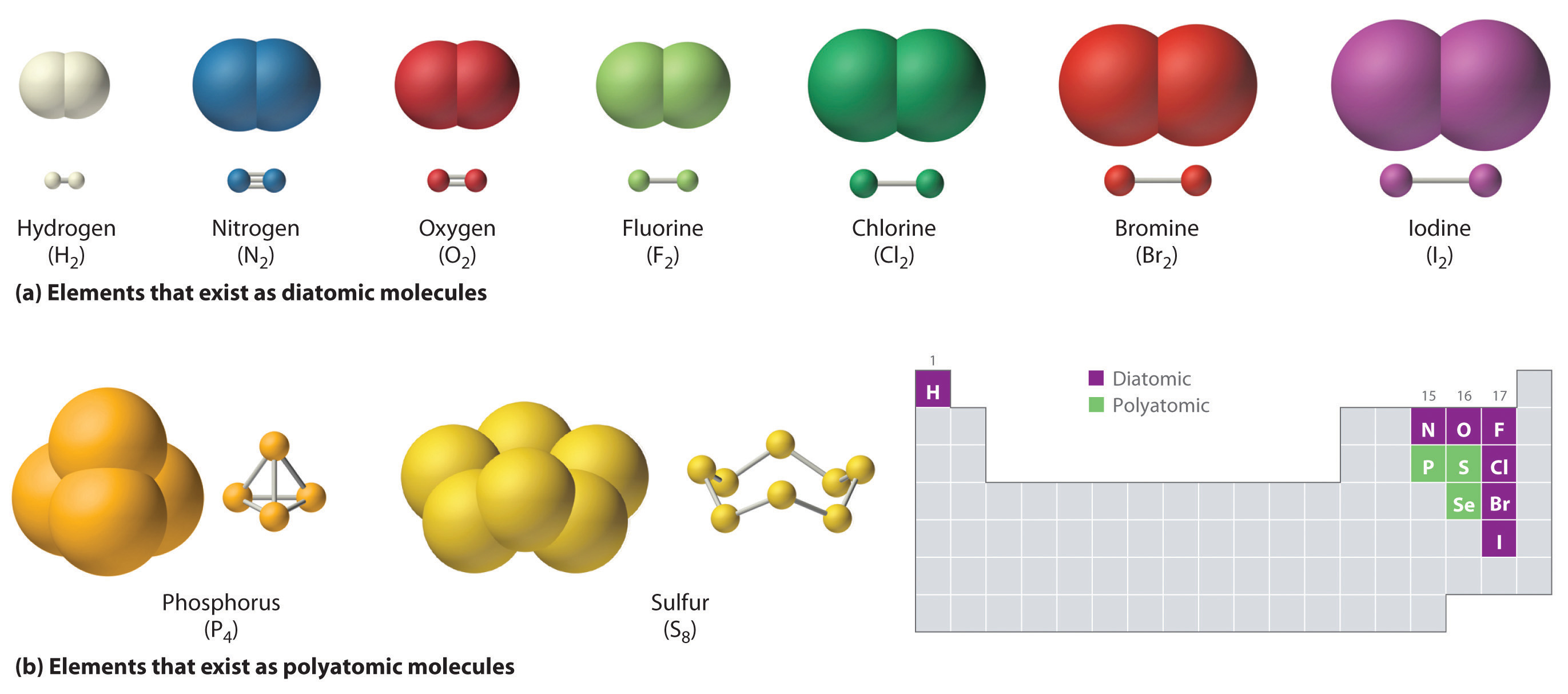
How Many Covalent Bonds Can Phosphorus Form - Group 6a form 2 bonds; If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be shared. Information starts using one straightforward picture off the standalone covalent bond. Web how many bonds does phosphorus typically make? The potential energy of two separate hydrogen atoms (right) decreases as.
Web as such, the \(\ce{s}\) atom must have 12 valence shell electrons to form 6 covalent bonds. Group 5a form 3 bonds; Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web this page explains what covalent bonding is. Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. And group 7a form one bond. Group 6a form 2 bonds;
covalent bond Definition, Properties, Examples, & Facts Britannica
Information starts using one straightforward picture off the standalone covalent bond. Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. Web (other elements, such as phosphorus [p] and cobalt [co], are able to form Valence shell electron pair repulsion theory (0) equatorial and.
Facts About Phosphorus Live Science
Web phosphorus is a nonmetallic element having an atomic number of 15. Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent. Web (other elements, such as phosphorus [p] and cobalt [co], are able to form Web the number of valence electrons in phosphorus is generally 5 and it requires.
Chemical Bonds · Anatomy and Physiology
Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. In each case, the sum. Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent. Is determined by the distance at which the lowest potential energy is achieved. Group 5a.
Reading Covalent Bonds Biology I
Group 6a form 2 bonds; Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent. If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be shared. An atom of any halogen, such.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Group 5a form 3 bonds; Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Covalent bond two atoms form a covalent chemical bond by each sharing at least one of their available valence. Is determined by the distance at which the lowest potential energy is achieved. And group 7a form one bond. This.
41. What is the Lewis dot structure of phosphate ion? how many
And group 7a form one bond. Similarly, the phosphorus atom in \(\ce{pcl_5}\) has 10 valence. Web typically, the atoms of group 4a form 4 covalent bonds; Is determined by the distance at which the lowest potential energy is achieved. If the atoms that form a covalent bond are identical, as in h 2, cl 2,.
Covalent bonding in an oxygen molecule. Chemistry Activities, Gcse
Web this page explains what covalent bonding is. Similarly, the phosphorus atom in \(\ce{pcl_5}\) has 10 valence. Web typically, the atoms of group 4a form 4 covalent bonds; Group 6a form 2 bonds; Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. If.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
It has 5 valence electrons, which means that it can form 3 covalent bonds.at most, the. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web molecular shapes & valence bond theory (0) worksheet. This is summarized in the table below. If the atoms.
Chemical Bonds · Anatomy and Physiology
If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be shared. Valence shell electron pair repulsion theory (0) equatorial and axial positions (0) electron geometry (0). Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images..
The Expanded Octet Introduction to Chemistry
Covalent bond two atoms form a covalent chemical bond by each sharing at least one of their available valence. Web the halogens also form single covalent bonds to produce diatomic molecules. It has 5 valence electrons, which means that it can form 3 covalent bonds.at most, the. Web typically, the atoms of group 4a form.
How Many Covalent Bonds Can Phosphorus Form Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be shared. Web the halogens also form single covalent bonds to produce diatomic molecules. Web this page explains what covalent bonding is. This is summarized in the table below.
An Atom Of Any Halogen, Such As Fluorine, Has Seven Valence Electrons.
If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be shared. It has 5 valence electrons, which means that it can form 3 covalent bonds.at most, the. Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. And group 7a form one bond.
Group 6A Form 2 Bonds;
Web covalent bonds involve the sharing of electron pairs between atoms. And group 7a form one bond. Web there are many different modifications of phosphorus in nature. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form.
Information Starts Using One Straightforward Picture Off The Standalone Covalent Bond.
Web molecular shapes & valence bond theory (0) worksheet. Web typically, the atoms of group 4a form 4 covalent bonds; This is summarized in the table below. Web the halogens also form single covalent bonds to produce diatomic molecules.
Web Typically, The Atoms Of Group 4A Form 4 Covalent Bonds;
Web this page explains what covalent bonding is. The potential energy of two separate hydrogen atoms (right) decreases as. Group 6a form 2 bonds; If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be shared.